World-Class Payloads Manufacturing
ADC Payloads Manufacturers in India
Pharmaceutical ADC Payloads Manufacturing and Supply
Shilpa Pharma is a world-class CDMO offering specialized, large-scale ADC payload manufacturing in India, focused exclusively on bulk production for global pharmaceutical and biotech innovators. Our state-of-the-art HPAPI and ADC payload facilities are fully cGMP-compliant and accredited by leading international regulatory authorities, including USFDA, EU GMP, ANVISA, COFEPRIS, TGA, PMDA, and KFDA. These approvals ensure that our facilities meet the highest global standards for safety, quality, and operational excellence, enabling the production of high-purity, highly potent ADC payloads suitable for both clinical and commercial requirements.
As a trusted partner for ADC payload development and bulk supply, we provide complete support — from process optimization, analytical characterization, and solid-state engineering to scalable manufacturing and secure global shipment. Our deep expertise in cytotoxic small-molecule warheads, combined with robust quality systems and regulatory-ready documentation, ensures seamless progress for your ADC programs.
Choose Shilpa Pharma for world-class ADC payload manufacturing in India, where accredited facilities, uncompromised precision, safety, and bulk-scale reliability power your commercial and clinical pipelines.
Complete Range of ADC Payloads
| Product Name | CAS No | Category / Mechanism | Enquire |
|---|---|---|---|
| DM1 | 139504-50-0 | Microtubule Inhibitor (Maytansinoid family) | Enquire Now |
| DM4 | 796073-69-3 | Microtubule Inhibitor (Maytansinoid family) | Enquire Now |
| Exatecan | 171335-80-1 | DNA Topoisomerase I Inhibitor (Camptothecin derivative) | Enquire Now |
| Exatecan Mesylate | 169869-90-3 | DNA Topoisomerase I Inhibitor | Enquire Now |
| Irinotecan | 100286-90-6 | DNA Topoisomerase I Inhibitor | Enquire Now |
| MMAE | 474645-27-7 | Microtubule Inhibitor (Auristatin family) | Enquire Now |
| MMAF | 745017-94-1 | Microtubule Inhibitor (Auristatin family) | Enquire Now |
| SN-38 | 86639-52-3 | DNA Topoisomerase I Inhibitor (Active metabolite of Irinotecan) | Enquire Now |
| Topotecan | 123948-87-8 | DNA Topoisomerase I Inhibitor | Enquire Now |
List of Payload-Linker (PL)
| S. No | Product Name | CAS No | Enquire |
|---|---|---|---|
| 1 | VcMMAE (MC-Val-Cit-PAB-MMAE) | 646502-53-6 | Enquire Now |
| 2 | MC-Val-Cit-PAB-Exatecan | 2504068-28-2 | Enquire Now |
| 3 | Mc-VC-PAB-SN38 | 1801838-28-7 | Enquire Now |
| 4 | DM1-SMCC | 1613362-80-3 | Enquire Now |
Disclaimer
The content on this website is for general informational purposes only. While we strive for accuracy, Shilpa Pharma makes no warranties regarding the completeness, reliability, or suitability of the information. For detailed product specifications, regulatory documentation, or inquiries, please contact us directly.
Global Leaders in ADC Payloads Manufacturing
Choosing Shilpa Pharma means choosing reliability, quality, and innovation. We deliver not just products, but long-term value that strengthens your business at every step.

Globally Compliant Manufacturing
ADC payloads are manufactured in cGMP facilities compliant with USFDA, EU GMP, ANVISA, COFEPRIS, TGA, PMDA, and KFDA.

Integrated Payload Expertise
Strong expertise in payload antibody drug conjugate, payload linker, and process development enables scalable and safe cancer drug payload production.

High Potency & Purity Control
Advanced analytical systems ensure high-purity anticancer cytotoxic payloads with strict impurity and genotoxic risk control.
Solid-State & Particle Optimization
Customized particle size and solid-state control support efficient conjugation and downstream formulation success.
Reliable Global Supply
Recognized among leading ADC payloads suppliers, ensuring consistent and secure global delivery. A trusted ADC payload manufacturer in India.
Oncology-Focused Manufacturing
A proven cancer payload manufacturer and cancer payload supplier, delivering high-quality anticancer payloads for global oncology programs.
Shilpa Pharma’s Portfolio of ADC Payload Contract Manufacturing in India
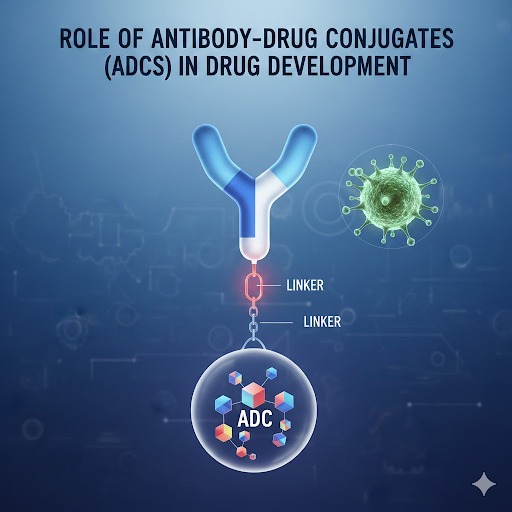
Shilpa Pharma offers a world-class and highly specialized portfolio of CDMO payload manufacturing in India, dedicated to large-scale and bulk production of high-potency ADC warheads. Our capabilities cover multiple categories of cytotoxic payloads, each manufactured under advanced containment, stringent quality systems, and global regulatory standards.
Our portfolio includes:
✔ Microtubule Inhibitor Payloads: Maytansinoids, auristatins, and related analogs designed for targeted inhibition of cancer cell division.
✔ DNA-Targeting Payloads: High-potency agents such as PBD dimers and calicheamicin derivatives, developed for precision DNA cross-linking and cell-killing activity.
✔ Topoisomerase Inhibitor Payloads: Payloads engineered for controlled DNA replication interference within the ADC mechanism.
✔ Proprietary & Custom Payloads: Co-development and bulk manufacturing of novel or client-specific small-molecule warheads tailored to unique ADC constructs.
Each payload class is supported by comprehensive analytical development, impurity profiling, stability assessment, and process optimization, ensuring consistent quality and reliable bulk supply. With world-class infrastructure and deep scientific expertise, Shilpa Pharma stands as a leading CDMO for ADC payload manufacturing in India, delivering scalable, safe, and commercially ready solutions for global ADC programs.
Shilpa Pharma: Leading ADC Payload Manufacturing Company in India
Shilpa Pharma is a preferred ADC payload manufacturing company in India for global biopharmaceutical partners, trusted for delivering high-quality ADC payloads that meet international regulatory and safety expectations. Our cGMP manufacturing facilities are compliant with USFDA, EU GMP, ANVISA, COFEPRIS, TGA, PMDA, and KFDA, enabling seamless supply to regulated markets worldwide.
With strong expertise in payload antibody drug conjugate, payload linker, and anticancer cytotoxic payload manufacturing, Shilpa Pharma combines scientific depth with operational excellence. Robust impurity control, advanced analytical systems, and scalable processes ensure consistent, formulation-ready cancer drug payloads. As a globally reliable ADC payload manufacturer in India, we offer international customers regulatory confidence, supply reliability, and cost-efficient manufacturing—making Shilpa Pharma a trusted cancer payload manufacturer and cancer payload supplier for worldwide oncology programs.
Explore Our Other Product Range
Get in Touch – Your Trusted Partner in ADC Payloads Manufacturing

Get a Quote
Happy Customers
Projects Successfully Completed

Established
PhD Experts Driving R&D Excellence
Team Size
World-Class API Manufacturing Sites
Global Audits Successfully Completed
Comprehensive CDMO Service Offerings
Questions & Answers
Our FAQ's
Everything You Need to Know About API Manufacturing in India at Shilpa Pharma
What ADC payload manufacturing capabilities does Shilpa Pharma offer?
Shilpa Pharma offers end-to-end ADC payloads manufacturing, including development and commercial-scale production of highly potent anticancer cytotoxic payloads, along with expertise in payload antibody drug conjugate and payload linker chemistry.
Is Shilpa Pharma compliant with global regulatory standards for ADC payload supply?
Yes. Shilpa Pharma’s facilities are cGMP-compliant and approved by USFDA, EU GMP, ANVISA, COFEPRIS, TGA, PMDA, and KFDA, enabling seamless global supply of cancer drug payloads to regulated markets.
Why choose an ADC payload manufacturer in India like Shilpa Pharma?
As a leading ADC payload manufacturer in India, Shilpa Pharma combines global regulatory compliance, advanced high-potency infrastructure, and cost-efficient manufacturing—making it an ideal partner for international oncology programs.
How does Shilpa Pharma ensure safety and quality in ADC payload production?
Advanced analytical controls, strict impurity and genotoxic risk management, and containment-driven manufacturing ensure consistent quality and safety across all ADC payloads and anticancer payloads.
Can Shilpa Pharma support clinical to commercial scale ADC payload supply?
Yes. Shilpa Pharma supports ADC programs from early clinical development through commercial supply, ensuring scalable, secure, and uninterrupted delivery as a trusted ADC payloads supplier.
What types of customers partner with Shilpa Pharma for ADC payloads?
Global pharmaceutical companies, biotech firms, and CDMOs partner with Shilpa Pharma as a reliable cancer payload manufacturer and cancer payload supplier for regulated international markets.
Does Shilpa Pharma support custom ADC payload manufacturing projects?
Yes. Shilpa Pharma offers customized ADC payload manufacturing, including process development, optimization, and scale-up tailored to specific payload antibody drug conjugate and cancer drug payload requirements.
How does Shilpa Pharma ensure supply chain security for international clients?
As a reliable ADC payloads supplier, Shilpa Pharma maintains validated supply systems, redundant sourcing, and controlled logistics to ensure uninterrupted global delivery of anticancer payloads.
What makes Shilpa Pharma a competitive alternative to Western ADC payload manufacturers?
Shilpa Pharma combines global regulatory compliance, advanced high-potency infrastructure, and cost-efficient manufacturing, positioning it as a preferred ADC payload manufacturer in India for international oncology programs.
Can Shilpa Pharma support long-term commercial supply agreements?
Yes. Shilpa Pharma supports long-term clinical and commercial supply agreements, offering consistent quality, regulatory continuity, and scalable capacity as a trusted cancer payload manufacturer and cancer payload supplier.