Shilpa Pharma Lifesciences Limited (SPLL) offers wide range of analytical chemistry services and offers best results with minimal timelines. These services could be either integrated with CDMO project or stand-alone project. Proven scientific capabilities, periodical communication and providing requisite data will be helpful to compile CMC documentation as required to support regulatory filings (IND/NDA) of our customers.
Shilpa Pharma has well qualified scientists and technical leaders who are dedicated in understanding analytical problem / requirements, deriving analytical solutions and serving our customers effectively.

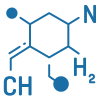
Structure elucidation is essential in pharmaceutical development for determining the exact molecular structure of compounds such as new chemical entities (NCEs), impurities, degradation products, and metabolites. At Shilpa Pharma, we use a range of advanced spectroscopic techniques—including NMR, mass spectrometry, IR, and UV-Visible spectroscopy—to accurately identify and confirm chemical structures. This supports product development, impurity profiling, and overall compound characterization.
These services play a critical role in ensuring product quality and regulatory readiness. By integrating structure elucidation with our broader analytical development services, we provide clear, reliable data to support method development, impurity evaluation, and compliance with global standards. Our work is carried out in a fully equipped analytical testing laboratory, helping accelerate CMC timelines while ensuring scientific accuracy and data integrity.
Read more




Systematic analysis of solid-state forms to identify potential polymorphs and assess stability characteristics.
Comprehensive screening and qualification of polymorphic forms to ensure optimal characteristics for NCE/API development.

Advanced analytical techniques used to quantify and establish the polymorphic nature in NCE/API formulations.
Forced degradation studies to showcase the specificity of stability indicating methods and gather essential data for determination of proper storage conditions, packaging and shelf life of product, which are vital for regulatory documentation
Establishing degradation pathways of drug substances


Revealing degradation mechanisms such as hydrolysis, oxidation, thermolysis or photolysis
Establishing degradation pathways of drug substances
Establishing the stability-indicating nature of a developed method
Understanding the chemical properties of drug molecules
Creating a degradation profile similar to formal stability studies under ICH conditions

Ensuring that a method developed in a client’s lab functions effectively at our lab, and vice versa, is crucial for maintaining consistency and meeting regulatory requirements. We focus on preserving precision throughout the entire life cycle of the API to ensure that the method is reliable, reproducible, and consistent across different laboratories or facilities.
Review the method and its development history (e.g., method validation results, data from the original lab).
Create a detailed AMT protocol outlining the transfer objectives, acceptance criteria, timeline, and test conditions.
Ensure that the receiving lab is equipped with the same or equivalent equipment to guarantee a seamless and effective process.
A detailed report documenting the method transfer process, including the results, deviations, and any corrective actions taken.

